
FDA’s Extended Review: Implications for Pediatric Atopic Dermatitis Treatment
The recent announcement by Incyte regarding the FDA’s extension of the review period for Opzelura, a topical treatment for atopic dermatitis in children aged 2-11, has significant implications for young patients and their families. For many parents, the frustration of watching their child struggle with a chronic skin condition can be overwhelming. This extension, now pushing the Prescription Drug User Fee Act action date to September 19, 2025, means there’s still hope for new treatment avenues amidst increasing numbers of children diagnosed with atopic dermatitis.
Understanding Atopic Dermatitis
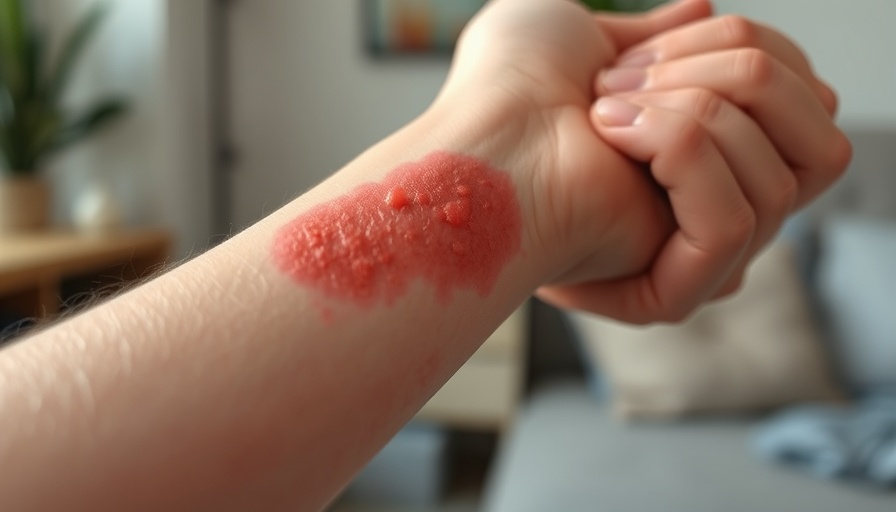
Atopic dermatitis is more than just skin deep; it's linked to immune system responses, and managing its symptoms can be challenging. Millions of children in the U.S. deal with this condition, leading to potential sleepless nights and discomfort. Steven Stein, MD, the chief medical officer at Incyte, emphasizes the importance of innovative treatments like ruxolitinib cream, which could offer relief without the steroids typically prescribed for such conditions.
Positive Results from Clinical Trials
The optimism surrounding Opzelura stems from its Phase 3 TRuE-AD3 study, which showcased significant treatment success compared to vehicle (non-medicated) controls. In the study, higher rates of treatment efficacy were recorded, which is essential information for parents seeking effective solutions for their children’s condition. Potential new remedies that minimize reliance on steroids could pave the way for enhanced treatment options.
Addressing Safety Concerns
When it comes to any new treatment, the safety profile is paramount. Opzelura demonstrated no new safety signals during the trial, and it did not lead to severe adverse events. Learning that the most common side effect—application site pain—was mild and transient offers reassurance to parents exploring treatment options for their young ones.
Why Parents Should Stay Informed
As a parent, staying informed about new treatments like Opzelura can be invaluable. Understanding available options, such as which pediatric plastic surgery specialists are trusted for related conditions or how to navigate cosmetic approaches, can empower parents to make informed health decisions for their children. As the FDA continues to evaluate Opzelura's application, the hope is that it will lead to new, safe, and effective treatments for pediatric atopic dermatitis, reducing the hardship many families face daily.
 Add Row
Add Row  Add
Add 



 Add Row
Add Row  Add
Add 

Write A Comment