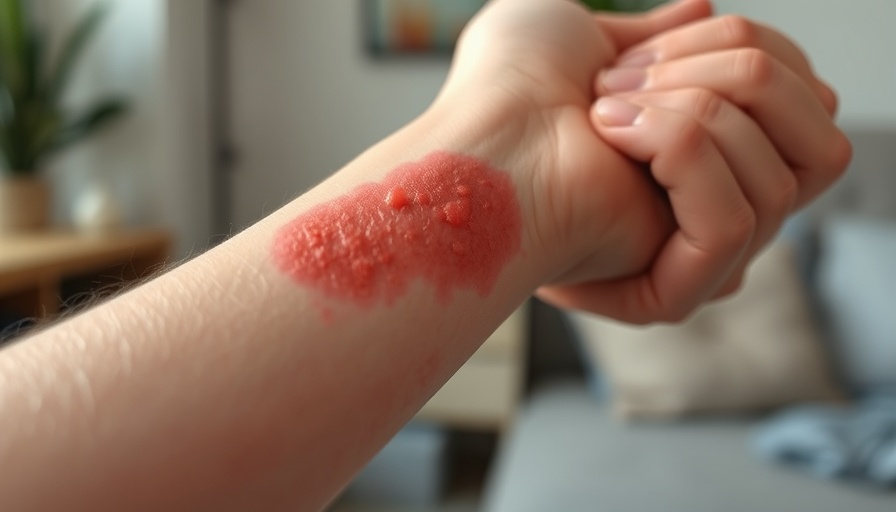
Opzelura: A Promising Treatment for Pediatric Atopic Dermatitis
The recent extension by the FDA regarding the review of Incyte’s ruxolitinib cream, branded as Opzelura, for treating pediatric atopic dermatitis has sparked both concern and hope among families affected by this chronic skin condition. With millions of American children suffering from atopic dermatitis, effective treatments are crucial, especially for parents seeking a balance between efficacy and safety in options available to their children.
Understanding Atopic Dermatitis
Atopic dermatitis is an immune-mediated condition characterized by dry, itchy skin, and can significantly impact a child's quality of life. According to Steven Stein, MD, chief medical officer at Incyte, this condition can be particularly challenging to manage, making the availability of new, safe, topical therapies incredibly valuable.
What's Next for Opzelura?
The FDA's new deadline for the action date is September 19, 2025, allowing more time to analyze additional data related to the cream’s manufacturing processes and safety. This extension also highlights the rigorous standards involved in bringing a medication to market, particularly for children.
Positive Results from Clinical Studies
The supplemental New Drug Application (sNDA) for Opzelura is supported by promising results from the Phase 3 TRuE-AD3 study, which evidenced a significant improvement in patients treated with Opzelura compared to a placebo. These findings are encouraging, indicating that this medication could offer a non-steroidal option that many are desperately seeking.
The Safety Profile of Opzelura
Moreover, the study revealed a favorable safety profile, with common adverse events being mild and manageable. In all, there were no serious infections or significant side effects reported during the trials. This raises hopes for parents worried about the long-term implications of their child's treatment.
Importance of Ongoing Research
As discussions around Opzelura continue in medical circles, it is essential for parents and caregivers to stay informed, understanding both the potential benefits and risks associated with this treatment. The delay does not mean a denial; rather, it is an opportunity for thorough scrutiny, ensuring that when approved, the medication is safe for our youngest patients.
Looking Forward to Future Treatments
The discussions around Opzelura address a larger conversation about pediatric treatments and the urgency of having safe, effective options. As the healthcare landscape evolves, staying up-to-date with emerging treatments continues to be crucial for those supporting children with health challenges.
 Add Row
Add Row  Add
Add 




 Add Row
Add Row  Add
Add 

Write A Comment